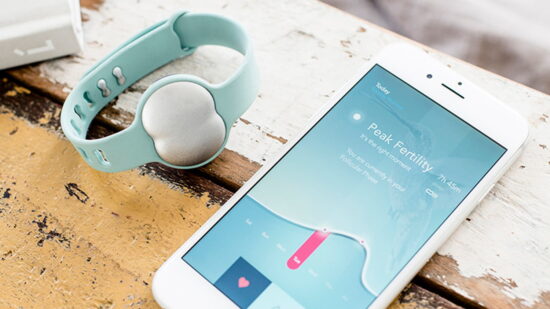
Ava, a digital healthcare company focused on women’s reproductive health, announced today it has received FDA clearance for its Ava Fertility Tracker, making it the first and only FDA-cleared fertility tracking wearable.
A fertility tracking sensor bracelet and accompanying app, Ava Fertility Tracker is already the first and only wearable, machine learning device to aid women in ovulation prediction and facilitation of conception. The device was successfully certified under the new European Device Regulation (MDR). Now, backed by the FDA clearance of its 501(k) application, Ava can also point to the efficacy of the Ava Fertility Tracker as comparable to time-trusted methods.
To gain FDA clearance, Ava submitted multiple performance tests, including a prospective clinical trial demonstrating that the Ava Fertility Tracker accurately predicted the day of ovulation. Results demonstrated that the Ava Fertility Tracker predicted the day of ovulation in a woman’s cycle just as well as urinary LH tests. The research also showed that the Ava Fertility Tracker detected significantly more post-ovulatory temperature shifts than other publicly available temperature-based ovulation detection products.
“The FDA clearance validates Ava’s efficacy as comparable or superior to standard products found in every pharmacy,” explains Ava Chief Medical Officer Maureen Cronin.
“However, Ava offers significant advantages over other fertility tracking methods. Not only is Ava easier and less invasive than having to pee on multiple sticks throughout the month, we also detect more fertile days in a woman’s cycle, giving her more time (and increased chances) to conceive. For example, LH tests only provide around 24 hours advance warning of ovulation, even though a woman is most fertile the three days preceding ovulation. Ava allows users to take advantage of more of the fertile window.”
In addition, according to Cronin, “Temperature-based methods of fertility tracking only identify ovulation after the fact, when a woman is no longer fertile. And many temperature-based methods of fertility tracking require that a woman wake up at the same time every morning to take her temperature. As a wearable, Ava collects temperature data – jointly with various other physiological parameters – much more conveniently. And because it measures temperature continuously throughout the night, our data showed that it is more sensitive to detecting post-ovulatory temperature shifts.”
Note: The Ava bracelet is not intended to be used for contraception.
About Ava
Ava is a digital health company with offices in Zurich, San Francisco, Belgrade and Makati that advances women’s reproductive health by bringing together artificial intelligence and clinical research. Its fertility tracking sensor bracelet detects the five most fertile days of a woman’s cycle in real-time with 90% accuracy, while also delivering personalized insight about reproductive health and pregnancy. Worn only during sleep, the Ava bracelet tracks multiple physiological parameters including pulse rate, breathing rate, and skin temperature. Launched in the US in 2016, the Ava bracelet is now sold in 36 countries and has helped around 40,000 women become pregnant. Studies for the clinical use of Ava have been conducted in collaboration with academic partners around the world, including at the University Hospital of Zurich, Switzerland, Columbia University, and Northwestern University’s Feinberg School of Medicine. Ava was voted Best of Baby Tech at CES, named a Women’s Health “Editors’ Choice” product and has been honored as a CB Insights Digital Health 150 company. For more information on Ava, visit www.avawomen.com.